/home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/861/header-lBanner.php on line 27
https://www.wahmg.com/)">
https://www.wahmg.com/)">
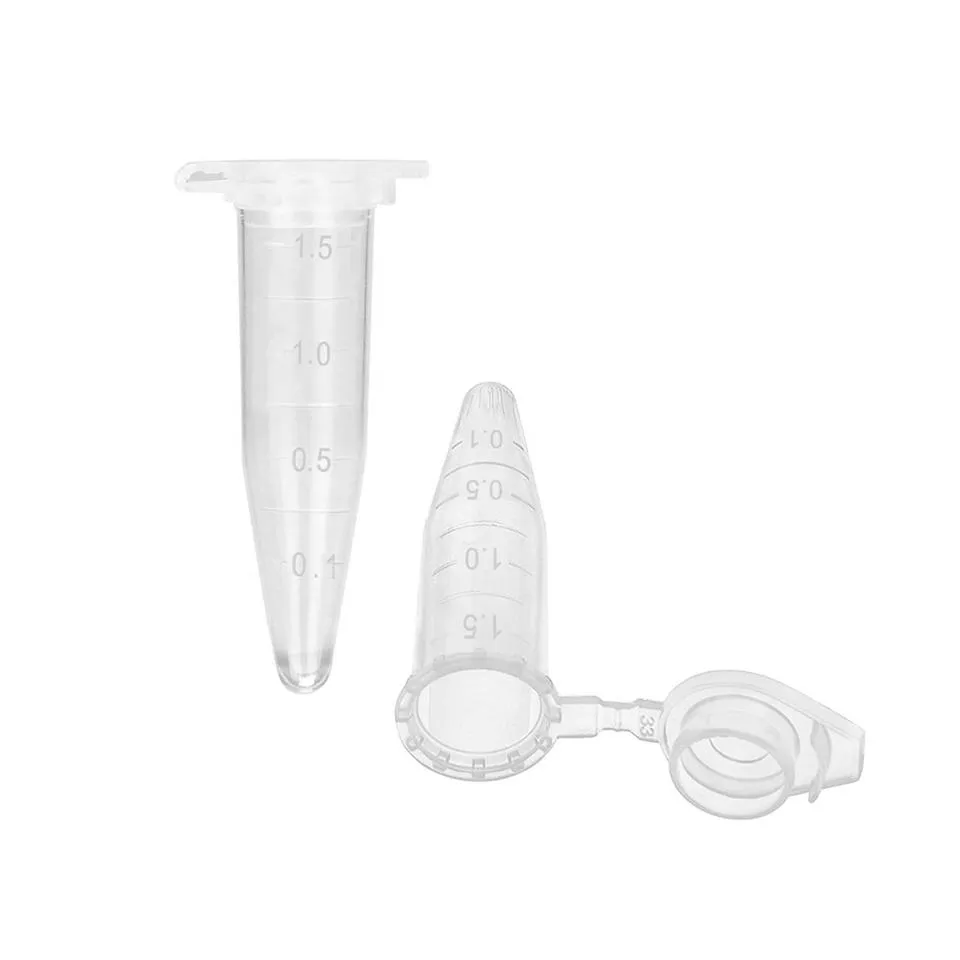
Plastic Freezer Tube For Laboratory Freezer Tube Beautiful And Customizable
1 月 . 15, 2025 09:49
Back to list
Plastic Freezer Tube For Laboratory Freezer Tube Beautiful And Customizable
EDTA vials are a crucial component in the world of medical and laboratory testing, yet often overlooked by those outside the health sciences. EDTA, which stands for Ethylenediaminetetraacetic acid, is a chelating agent used to sequester metal ions in solution. In lab settings, EDTA's ability to bind with calcium, magnesium, and iron ions makes it an invaluable tool, particularly in the preservation and stabilization of blood during testing. Understanding its role and function can be essential for anyone involved in clinical diagnostics or medical research.
The credibility and trustworthiness of the EDTA vial also lie in its proven track record. Clinicians and lab technicians have long relied on the precision and stability provided by EDTA in managing and diagnosing various health conditions, including anemia, infection, and hematology disorders. The predictable and reliable results underscore the necessity of such a tool, granting confidence to all involved parties, from lab technicians to healthcare providers and ultimately, patients. When choosing the most suitable EDTA vials for your practice or laboratory, consider factors such as tube volume, the concentration of EDTA, and the manufacturer’s quality assurance certifications. It is important to source vials from reputable companies that comply with global healthcare standards, ensuring that each unit adheres strictly to quality controls. The experience with using these vials also highlights the need for proper training for all personnel involved in sample collection and processing to maximize the advantages of using EDTA as an anticoagulant. In conclusion, the comprehensive understanding of EDTA vials’ role and application is paramount for those involved in clinical and laboratory settings. Through expertise, authoritative, and trustworthy practices, professionals are equipped to deliver accurate and efficient diagnostics. EDTA vials remain a linchpin in preserving the quality and integrity of blood samples, contributing significantly to advancing medical diagnostics and research.


The credibility and trustworthiness of the EDTA vial also lie in its proven track record. Clinicians and lab technicians have long relied on the precision and stability provided by EDTA in managing and diagnosing various health conditions, including anemia, infection, and hematology disorders. The predictable and reliable results underscore the necessity of such a tool, granting confidence to all involved parties, from lab technicians to healthcare providers and ultimately, patients. When choosing the most suitable EDTA vials for your practice or laboratory, consider factors such as tube volume, the concentration of EDTA, and the manufacturer’s quality assurance certifications. It is important to source vials from reputable companies that comply with global healthcare standards, ensuring that each unit adheres strictly to quality controls. The experience with using these vials also highlights the need for proper training for all personnel involved in sample collection and processing to maximize the advantages of using EDTA as an anticoagulant. In conclusion, the comprehensive understanding of EDTA vials’ role and application is paramount for those involved in clinical and laboratory settings. Through expertise, authoritative, and trustworthy practices, professionals are equipped to deliver accurate and efficient diagnostics. EDTA vials remain a linchpin in preserving the quality and integrity of blood samples, contributing significantly to advancing medical diagnostics and research.
Share
Latest news
-
Wholesale Plastic Juice Bottles with Caps 16 oz Options Available Bulk Packaging SolutionsNewsJun.10,2025
-
Laboratory Apparatus Reagent Bottle – Durable & Chemical Resistant Bottles for Safe StorageNewsJun.10,2025
-
Squeezable Dropper Bottles Durable, Leak-Proof & CustomizableNewsMay.30,2025
-
Affordable Plastic Petri Plates Sterile & Disposable Lab-GradeNewsMay.30,2025
-
Eye Dropper Caps Precision 24/410 & Plastic Bottle-Compatible TipsNewsMay.30,2025
-
Affordable Mini Spray Bottle Price & Wholesale Deals Shop NowNewsMay.29,2025
RECOMMEND PRODUCTS